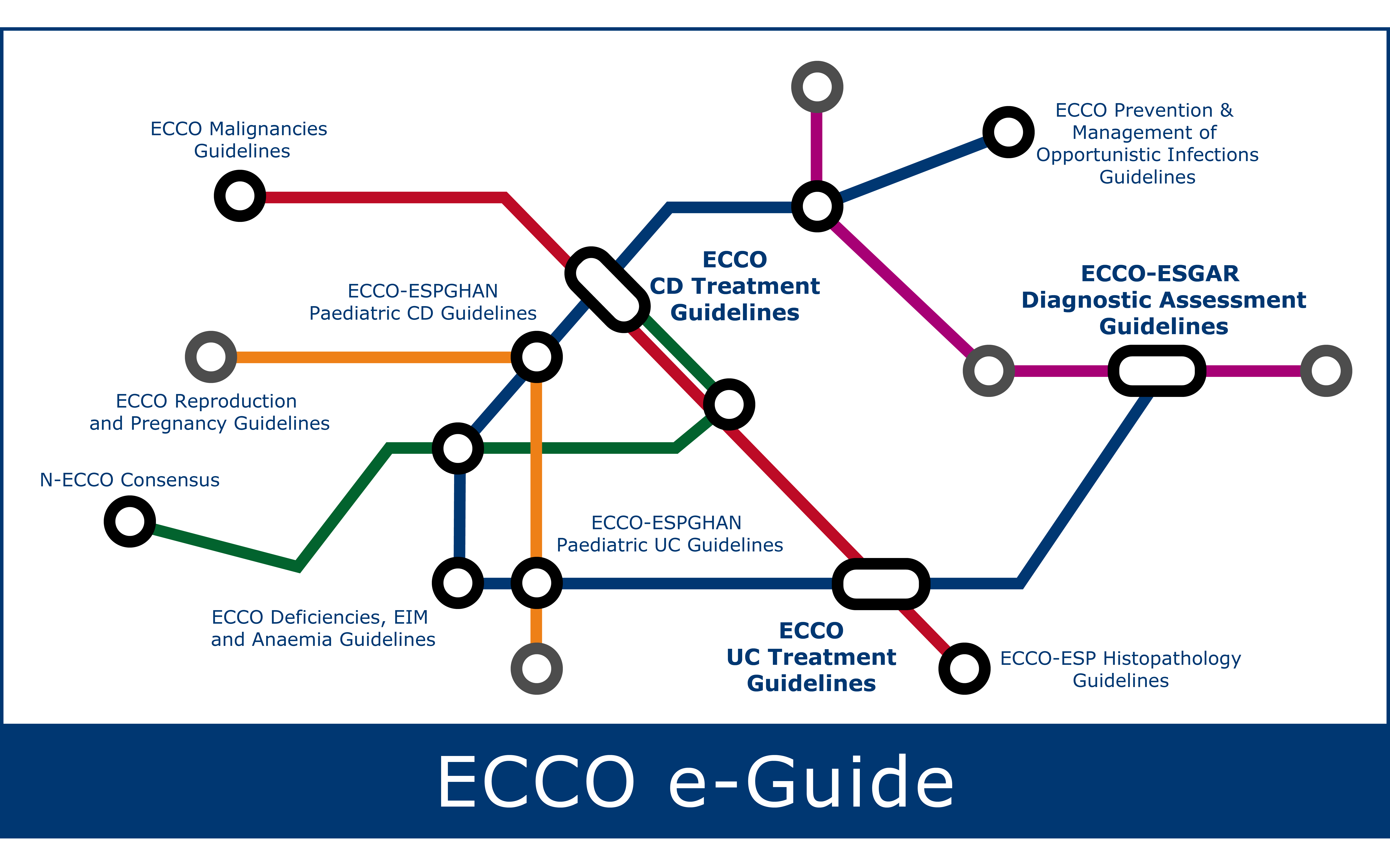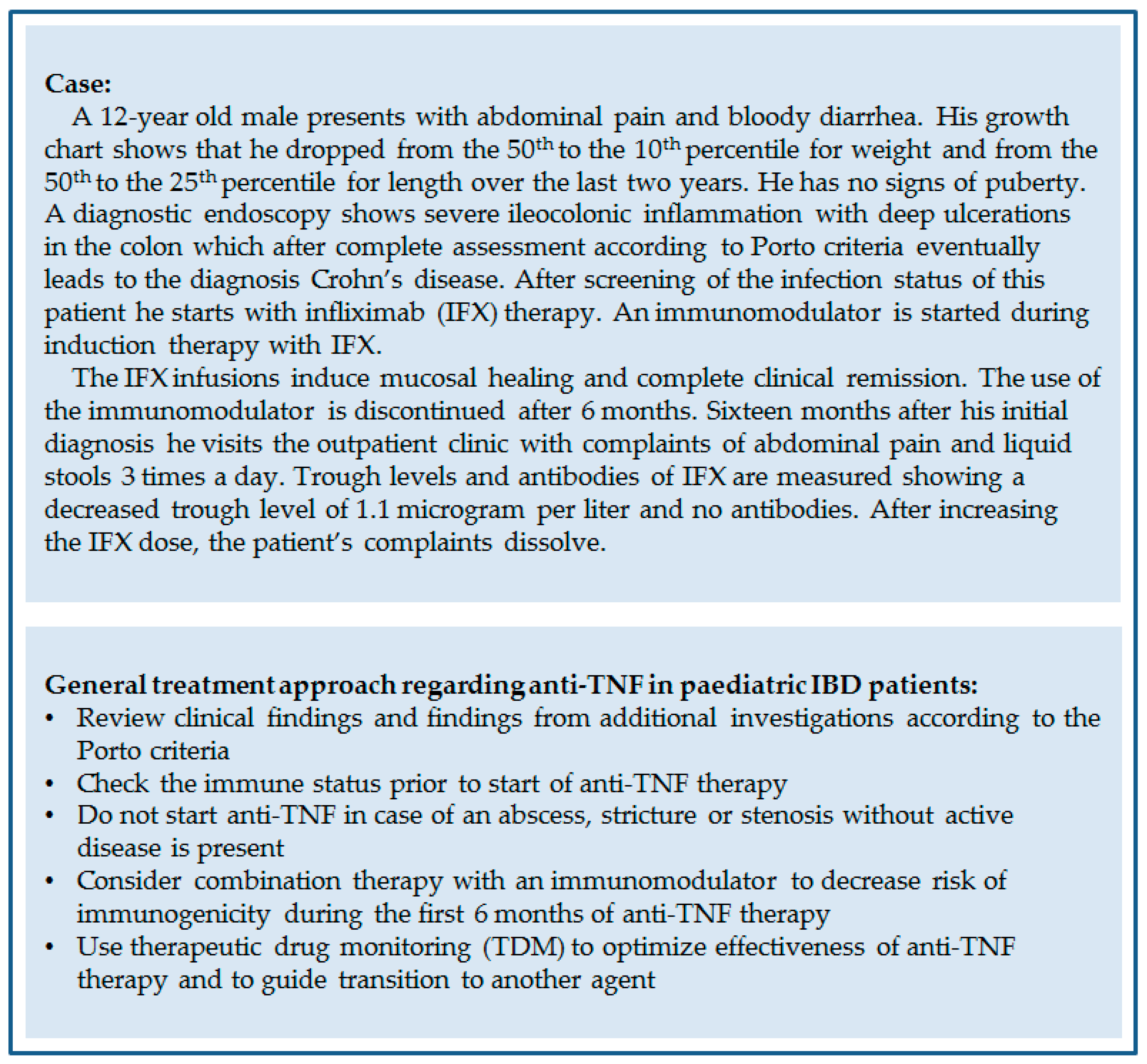
IJMS | Free Full-Text | A Review on the Use of Anti-TNF in Children and Adolescents with Inflammatory Bowel Disease

European Crohn´s and Colitis Organisation - ECCO - OP21 Positivity thresholds of total infliximab and adalimumab anti-drug antibody assay: The prevalence of clearing and transient anti-drug antibodies in a national therapeutic drug

IJMS | Free Full-Text | The Role of TNF-α and Anti-TNF-α Agents during Preconception, Pregnancy, and Breastfeeding
PDF) Biologics recommendations in the ECCO guidelines on therapeutics in Crohn's disease: Medical treatment
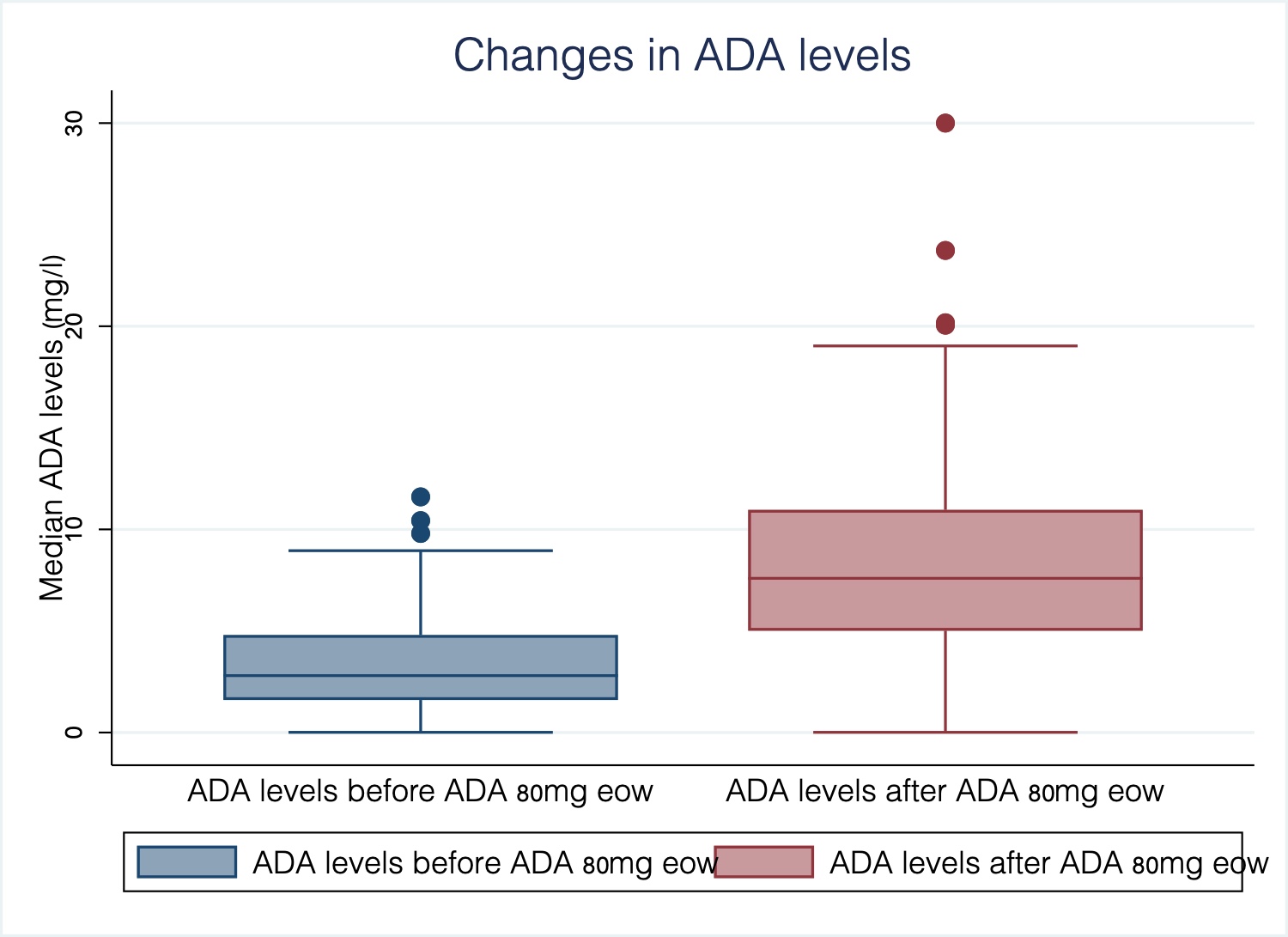
European Crohn´s and Colitis Organisation - ECCO - P533 Adalimumab 80mg every other week in inflammatory bowel disease: Treatment intensification outcomes in real life clinical practice

PDF) Adalimumab Monotherapy and a Combination with Azathioprine for Crohn's Disease: A Prospective, Randomized Trial

PDF) P553 Efficacy, safety and cost-efficiency of adalimumab 80 mg every other week in previously intensified IBD patients under treatment with adalimumab 40 mg every week
![PDF] Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. | Semantic Scholar PDF] Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/434bc5078bbc62a1a4782f27592fcf28f239679e/8-Table2-1.png)
PDF] Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. | Semantic Scholar
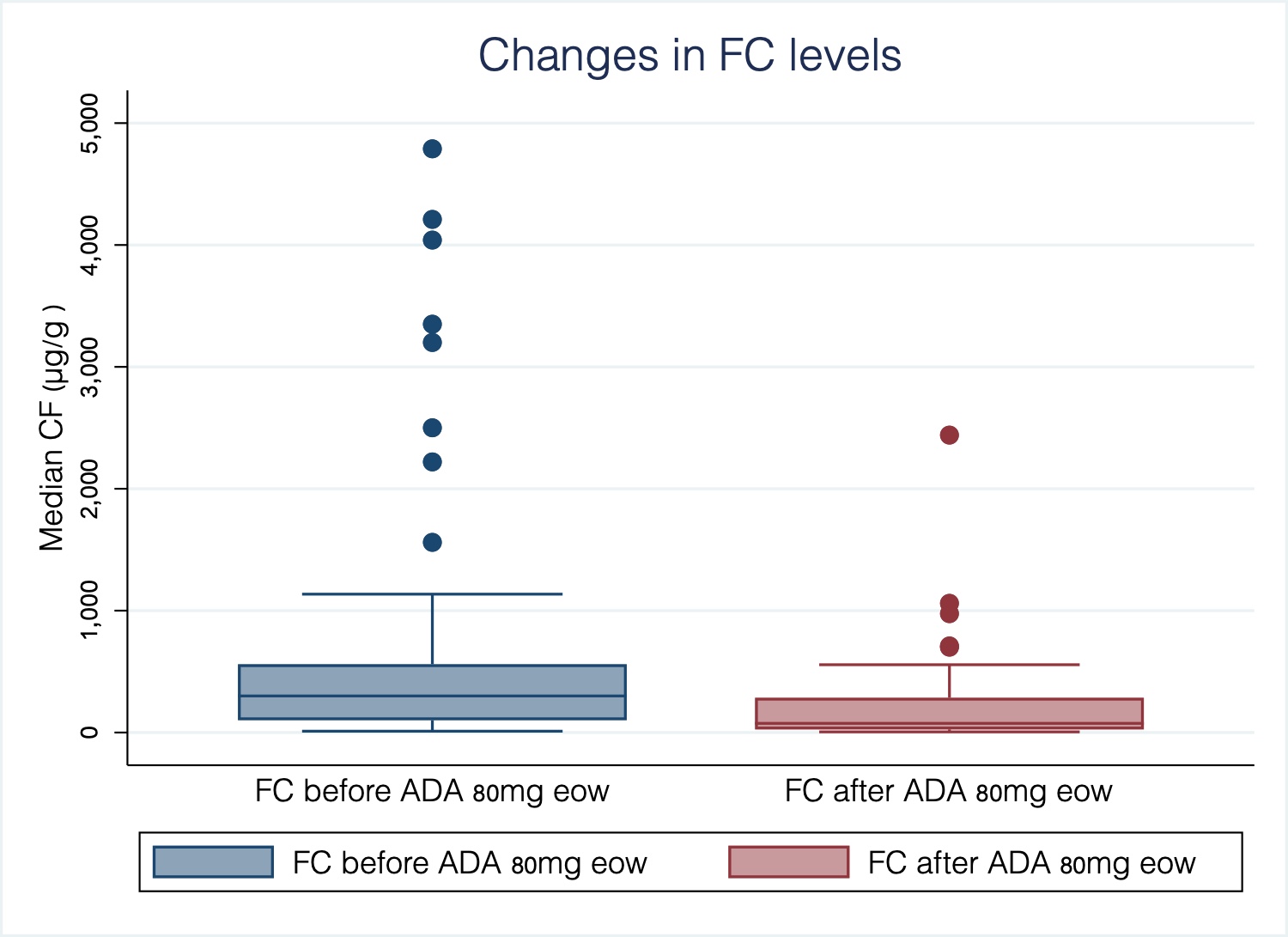
European Crohn´s and Colitis Organisation - ECCO - P533 Adalimumab 80mg every other week in inflammatory bowel disease: Treatment intensification outcomes in real life clinical practice

Biologics recommendations in the ECCO guidelines on therapeutics in Crohn's disease: medical treatment | Frontline Gastroenterology

Increased versus conventional adalimumab dose interval for patients with Crohn's disease in stable remission (LADI): a pragmatic, open-label, non-inferiority, randomised controlled trial - The Lancet Gastroenterology & Hepatology

PDF) Efficacy and Safety of Adalimumab by Disease Duration: Analysis of Pooled Data From Crohn's Disease Studies

Rational Combination Therapy to Overcome the Plateau of Drug Efficacy in Inflammatory Bowel Disease - Gastroenterology
Treatment Algorithms for Crohn's Disease - FullText - Digestion 2020, Vol. 101, Suppl. 1 - Karger Publishers
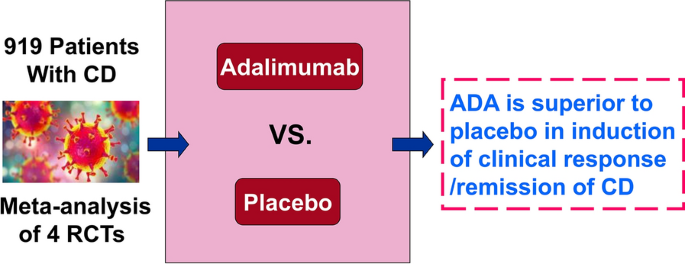
Adalimumab for induction of remission in patients with Crohn's disease: a systematic review and meta-analysis | European Journal of Medical Research | Full Text
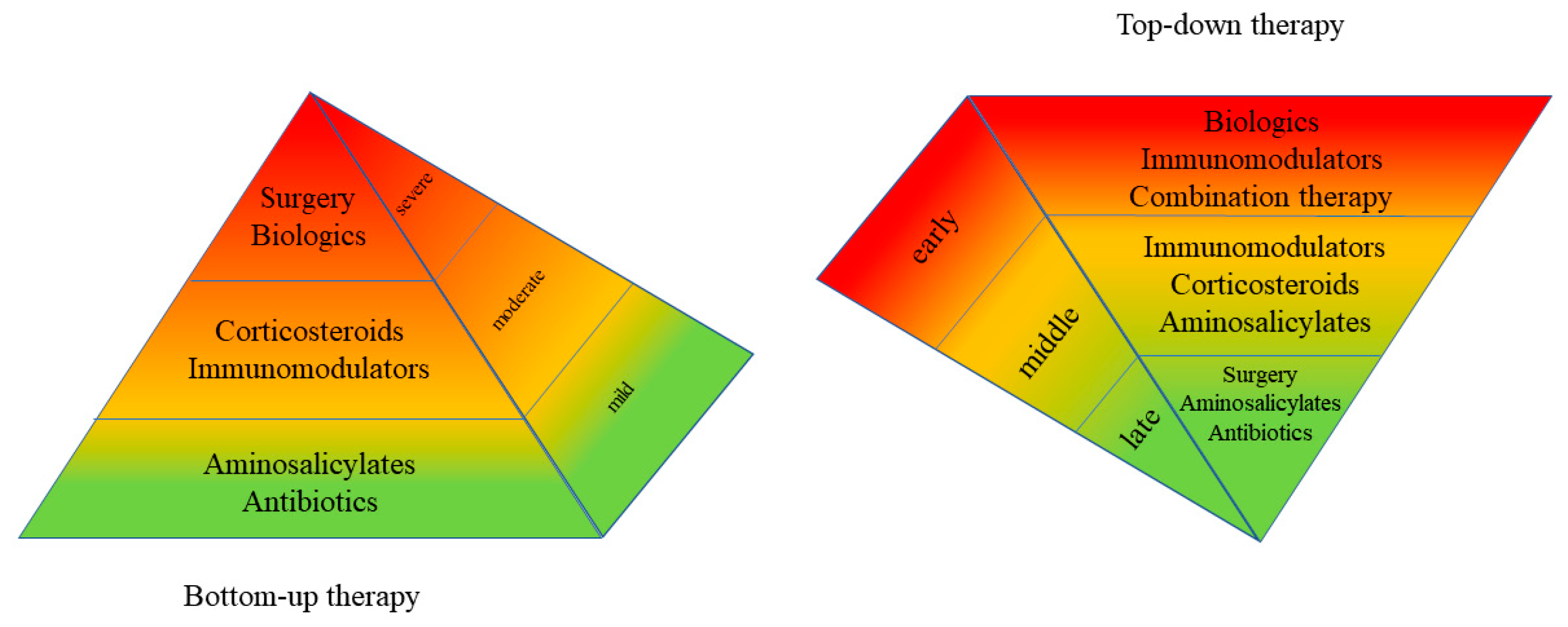
Biomedicines | Free Full-Text | The New Proactive Approach and Precision Medicine in Crohn's Disease

Dynamics of circulating TNF during adalimumab treatment using a drug-tolerant TNF assay | Science Translational Medicine

European Crohn´s and Colitis Organisation - ECCO - P533 Adalimumab 80mg every other week in inflammatory bowel disease: Treatment intensification outcomes in real life clinical practice